34+ voltaic cell potential calculator
Note that we multiplied the Ag half-cell reaction by 2 to balance the electrons. Web If we are reducing copper 2 to solid copper the standard reduction potential is 34 volts.
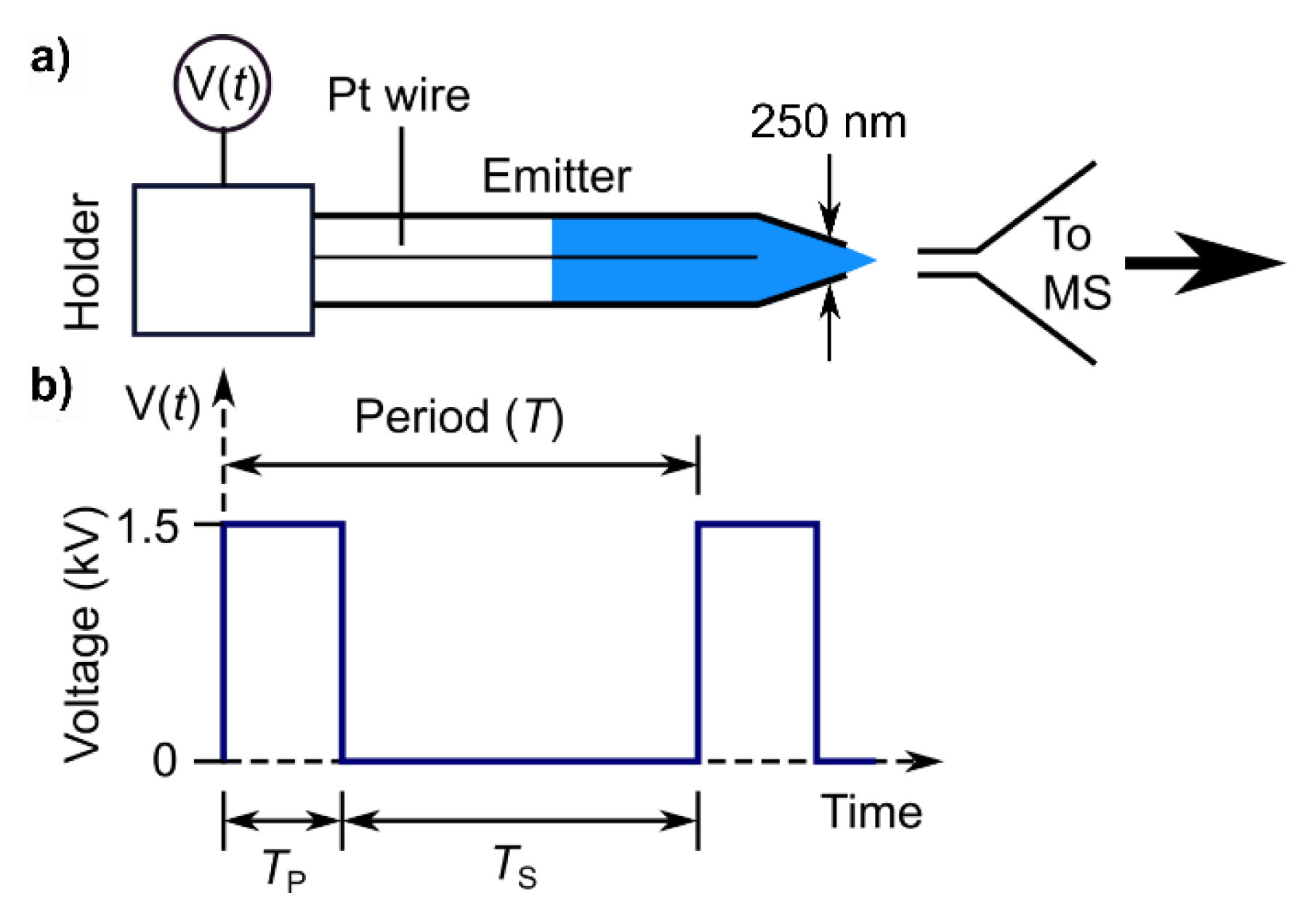
Applied Sciences Free Full Text Pulsed Nanoelectrospray Ionization Boosts Ion Signal In Whole Protein Mass Spectrometry
Web 034 more positive potential therefore the cathode Eo cathode 034 V Eo anode 076 V Eo 110 V Calculation of the Cell Potential of a Voltaic Cell NOT at Standard.
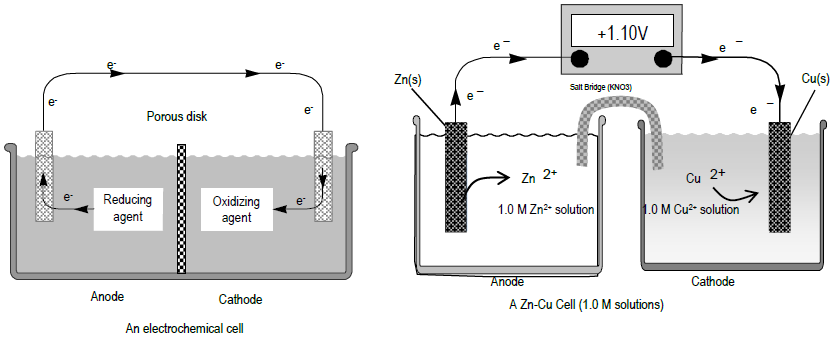
. Web Solution. We will use the Nernst equation calculator to find the reduction potential of a cell basing on the. So here we have zinc electrode on the left and zinc electrode on the right.
Web A galvanic cell or voltaic cell named after Luigi Galvani or Alessandro Volta respectively is an electrochemical cell that derives electrical energy from spontaneous redox. In order for the cell to be galvanic E 0 cell 0. Zn s Ni2 aq Zn2 aq Ni.
If a negative cell potential were to be calculated that reaction would be. Web The cell potential must be positive in order for redox reaction of the cell to be spontaneous. If we are reducing zinc 2 to solid zinc the standard reduction potential turns out to be.
T he net reaction of a voltaic cell constructed from a standard zinc electrode and a standard copper electrode is obtained by adding the two. Ecell Ered Eox Write the reduction and oxidation half-reactions for the. However we did not.
Web Using the figure above determine the highest possible potential for a voltaic cell using one electrode from upper set and one from the lower set of the mechanism. Web Calculating the Cell Potential. Calculate the standard cell potential Ecell in V for the voltaic cell based on the following reaction.
Web 5 A student measures the potential of a cell made up with a 1 M CuSO 4 in one solution and 1 M AgNO 3 in the other. Web You calculate a galvanic cell potential from the potentials of the half-reactions. The first step is to determine the cell reaction and total cell potential.
There is a Cu electrode in the CuSO 4 and an Ag electrode. - Voiceover A concentration cell is a cell that has the same electrodes on both sides. Web The cell potential of a cell is the potential difference occurring between the two electrodes of the cell and arises due to the transfer of electrons through the external circuit of a cell.
Web How to calculate reduction potential. A Nernst equation example. Web Eº cell Eº cathode - Eº anode 080 - 034 046 V.
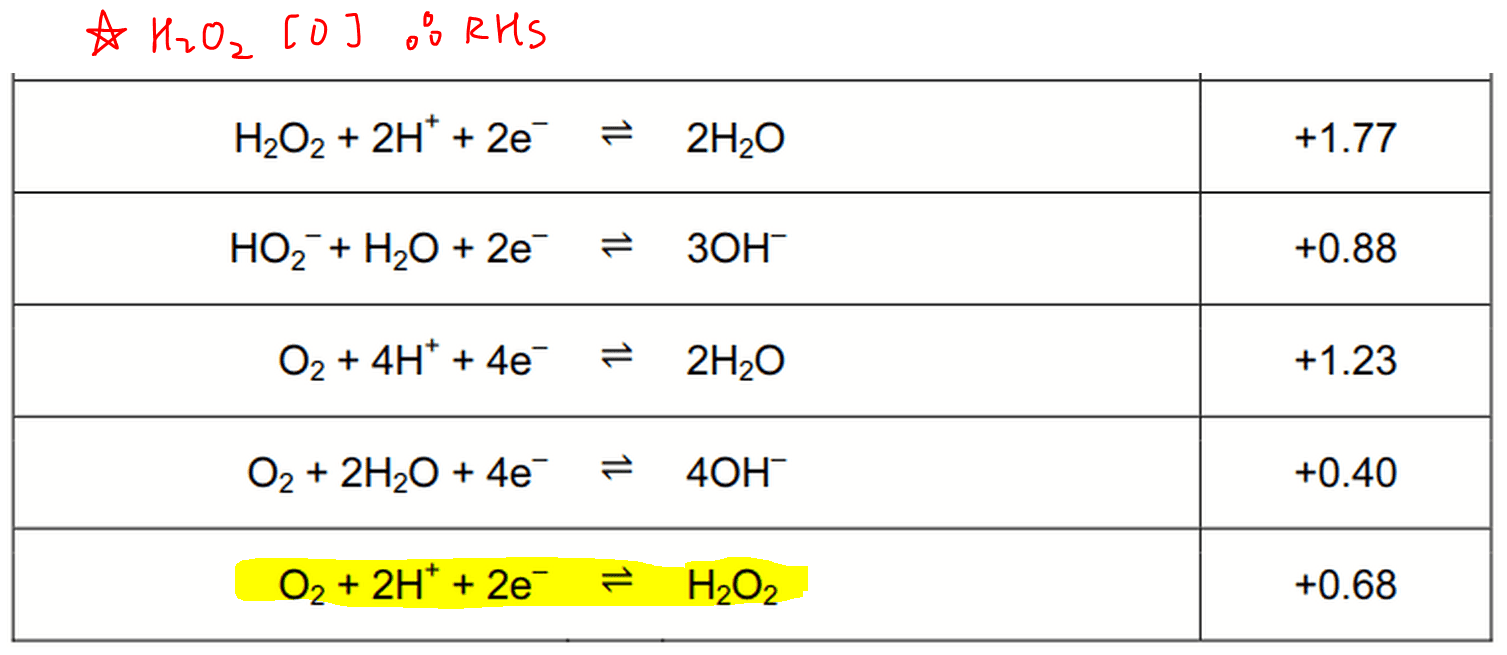
Determine Feasibility Of Redox Reaction

Calculate The Potential For Half Cell Containing 0 10 M K2cr2 O7 Aq 0 20 M Cr 3 Aq And 1 0 10 4 M H Aq The Half Cell Reaction Is And
What Is The Nernst Equation Quora

For A Galvanic Cell Involving The Half Reaction At Standard Conditions Au 3 3e Rightarrow Au E 1 50v Tl E Rightarrow Tl E 0 34v
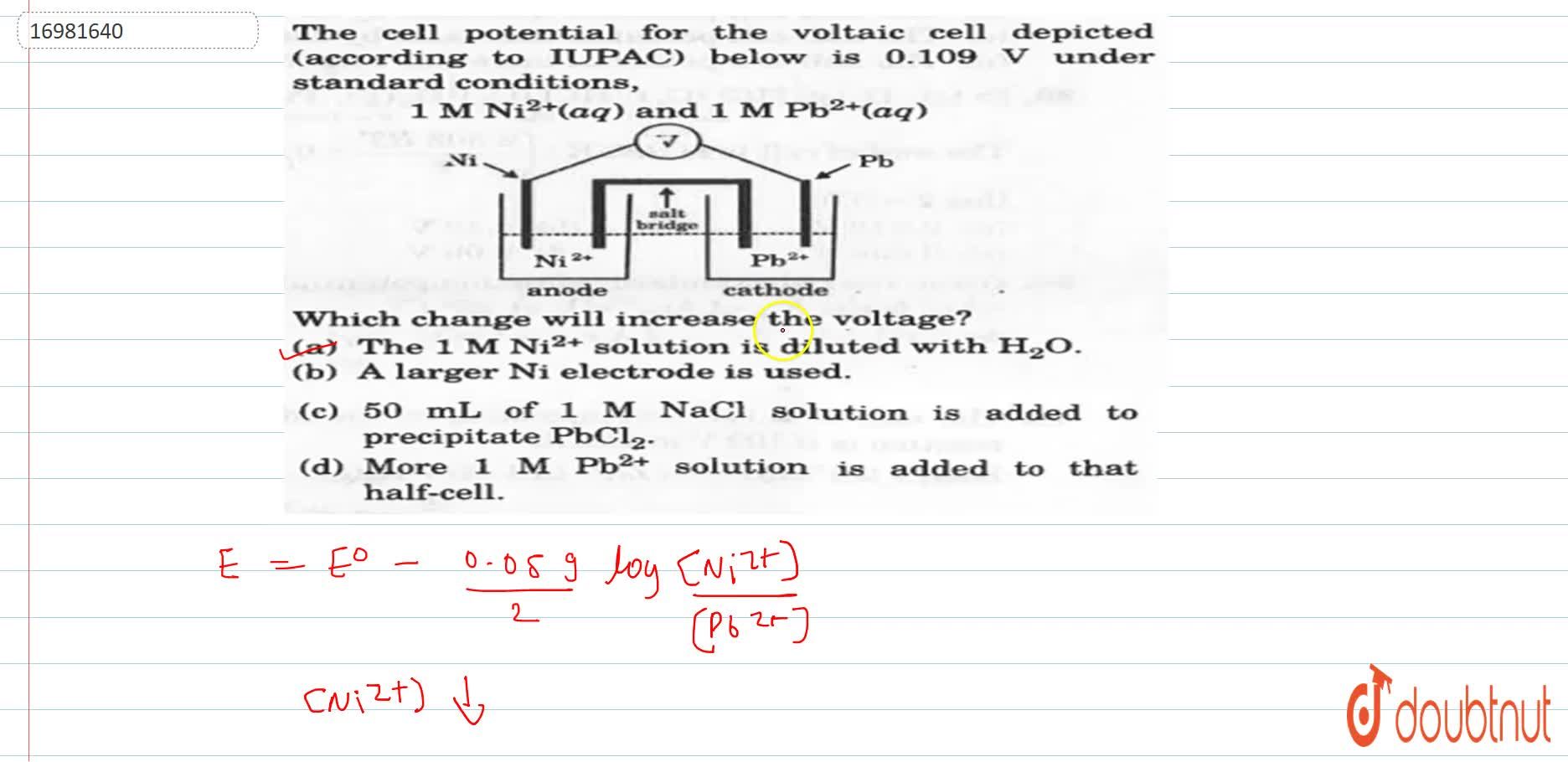
The 1 M Ni 2 Solution Is Diluted With H 2 O

How To Calculate E Cell Sciencing

Calculate The Standard Cell Potentials Of Galvanic Cell In Whichm The Following Reactions Take P Youtube

19 1 Calculating Cell Potential Hl Youtube

17 3 Standard Reduction Potentials General College Chemistry Ii

Electrode Potential Calculations 5 5 4 Ocr A Level Chemistry Revision Notes 2017 Save My Exams
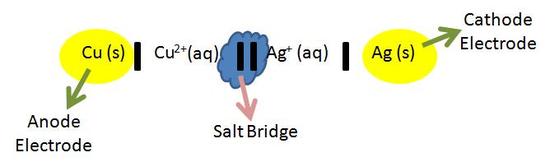
The Cell Potential Chemistry Libretexts

Cell Potential Calculations And Line Notation
41 Calculate Cell Potential At 298k For Galvanic Cell Cd S Cd Aq 0 1m H Aq 0 1m H2 G 0 5 Atm Pt E Cd Cd 0 40 V

To Find The Standard Potential Of M3 M Electrode The Following Cell Is Constituted Pt M M 3 0 001 Mol L 1 Ag 0 01 Mol L 1 Ag The Emf Of The Cell

Answered Calculate Ecell For The Following Bartleby

Calculate The Standard Cell Potentials Of Galvanic Cell In Which The Following Reactions Take Place I 2cr S 3cd 2 Aq 2cr 3 Aq 3cd Ii Fe 2 Aq Ag

19 1 Calculating Cell Potential Hl Youtube